








The fuel-cell was invented in 1839 by Sir William Robert Grove (1811-1896), a Welsh-born London lawyer and scientist. He and Christian Friedrich Schoenbein (1799-1868) are considered the fathers of fuel-cell technology. But problems with the materials and the invention of the internal-combustion engine and the electric generator prevented fuel-cells from becoming a reality. In 1874 Jules Verne predicted that the world would run on them. Sadly, that prediction has yet to be fulfilled. If it had been we would not be facing the global environmental catastrophe caused by the burning of fossil-fuels and other hydrocarbons.
Not till the1960s did fuel-cell technology start to come into its own, when space-
craft needed a compact, efficient power-source, and space administrations had the necessary wealth and research capacity. Now, as environmental degradation accelerates globally and fossil energy begins to run out, environmentally-friendly fuels and efficient energy-converters are coming into high demand. That drives fuel-cell R&D, and the law of supply and demand is bringing prices down.
Not till the1960s did fuel-cell technology start to come into its own, when space-
craft needed a compact, efficient power-source, and space administrations had the necessary wealth and research capacity. Now, as environmental degradation accelerates globally and fossil energy begins to run out, environmentally-friendly fuels and efficient energy-converters are coming into high demand. That drives fuel-cell R&D, and the law of supply and demand is bringing prices down.
In a fuel-cell, chemical energy from hydrogen and oxygen (pure oxygen or oxygen from the air) is directly converted to electrical energy--i.e., without a combustion process. All fuel-cells essentially consist of two electrodes (the plate-
like cathode and anode, the negative and positive electrodes) and the electrolyte (a medium that can transport the ions--charged subatomic particles) that separates the two electrodes from each other.
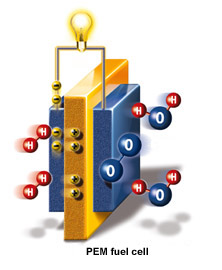
Fuel-cells are usually classified by the type of electrolyte used. The most common kind of fuel-
cell, the kind that by far dominates the market, is the PEM fuel-cell. PEM stands for proton-exchange membrane, which is a thin polymer film that allows hydrogen ions to pass through it but blocks electrons. Thus, when a hydrogen atom has its electron stripped off it at the anode plate in the fuel-cell (the positive plate) by the catalytic action of a small quantity of platinum, the proton (the
like cathode and anode, the negative and positive electrodes) and the electrolyte (a medium that can transport the ions--charged subatomic particles) that separates the two electrodes from each other.
Fuel-cells are usually classified by the type of electrolyte used. The most common kind of fuel-
cell, the kind that by far dominates the market, is the PEM fuel-cell. PEM stands for proton-exchange membrane, which is a thin polymer film that allows hydrogen ions to pass through it but blocks electrons. Thus, when a hydrogen atom has its electron stripped off it at the anode plate in the fuel-cell (the positive plate) by the catalytic action of a small quantity of platinum, the proton (the
nucleus) goes through the PEM but the electron has to go round the circuit to the cathode plate to rejoin it. That creates a current, which does electrical work, such as powering a light-bulb or an electric motor.
On the other side of the PEM (at the cathode plate, the negative electrode), the hydrogen combines with oxygen to form water. Some heat is also given off (80 to 90 degrees Celsius, or 176 to 194 degrees Fahrenheit ). So a fuel-cell consumes nothing but hydrogen and oxygen and gives off nothing but electricity, heat and 100% pure water. You can drink the exhaust.
In the diagram above left, the electrodes are coloured blue; the PEM electrolyte between them is orange. Hydrogen atoms are red and oxygen blue. Hydrogen ions are yellow with a positive sign on them; electrons are also yellow, with a negative sign. You can see on the right the hydrogen combining in pairs with oxygen to form pure water.
On the other side of the PEM (at the cathode plate, the negative electrode), the hydrogen combines with oxygen to form water. Some heat is also given off (80 to 90 degrees Celsius, or 176 to 194 degrees Fahrenheit ). So a fuel-cell consumes nothing but hydrogen and oxygen and gives off nothing but electricity, heat and 100% pure water. You can drink the exhaust.
In the diagram above left, the electrodes are coloured blue; the PEM electrolyte between them is orange. Hydrogen atoms are red and oxygen blue. Hydrogen ions are yellow with a positive sign on them; electrons are also yellow, with a negative sign. You can see on the right the hydrogen combining in pairs with oxygen to form pure water.
As with any battery, individual fuel-cells can be connected together to increase voltage. More than one fuel-cell combined in this way is called a 'stack', represented in the right-hand diagram above. The black shapes in that diagram are the plate-like electroodes, which have shallow grooves on both sides so that hydrogen and oxygen (or air) can flow along their surfaces. The output of a fuel-cell stack can be varied by increasing the number of cells in it, and by increasing their area. The more cells, the higher the voltage; the greater the cross-sectional area the higher the current. A big fuel-cell can generate hundreds of kilowatts and supply a whole neighbourhood. But big in power does not mean physically big. A 50kW PEM fuel-cell is only 350x350mm (14x14") in cross-section, 600mm long (24") and weighs 80kg (178 pounds).
Making the hydrogen to drive a fuel-cell is best done by splitting water in an electrolyser. That makes the perfect cycle: the electrolyser splits the water into hydrogen and oxygen; the fuel-cells joins them back into water. Hydrogen thus becomes a medium for storing electrical energy. The best source of that energy is from natural sources: sunlight, water and wind. Then nothing comes from the sky but sunlight, water and air; and nothing goes back into it except water, oxygen, and some heat.
Making the hydrogen to drive a fuel-cell is best done by splitting water in an electrolyser. That makes the perfect cycle: the electrolyser splits the water into hydrogen and oxygen; the fuel-cells joins them back into water. Hydrogen thus becomes a medium for storing electrical energy. The best source of that energy is from natural sources: sunlight, water and wind. Then nothing comes from the sky but sunlight, water and air; and nothing goes back into it except water, oxygen, and some heat.


A fuel-cell is a kind of battery. But, unlike other batteries, it does not store an electrical charge. Instead it has a tank of fuel--
hydrogen--from which it produces electricity by combining it with oxygen. So long as it is supplied with hydrogen it produces electricity. The exhaust is only pure water, which does not harm the planet.
hydrogen--from which it produces electricity by combining it with oxygen. So long as it is supplied with hydrogen it produces electricity. The exhaust is only pure water, which does not harm the planet.





